Industrial Consultancy & Sponsored Research (IC&SR) , IIT Madras
Microneedle Array Device and Method thereof
Categories for this Invention
Technology: Microneedle device with calibration mode
Category: Assistive, Test Equipment & Design Manufacturing
Industry: Biomedical
Application: Calibration of microneedle
Market: The global market size of was USD 47,040 million in 2021 and market is touch USD 105480.6 million by 2031 at CAGR 8.3% during the forecast period.
Image Gallery
Problem Statement
- Microneedle array devices (MA) are devices that can be configured to perform fluid delivery or extraction to or from a user’s skin.
- These devices can be configured to inject medication or perform fluid sampling, depending on the coupling of at least one microneedle with the user’s skin.
- The MA may have a control system that interacts with extracted fluid sampling to detect user conditions, such as diabetic conditions.
- In cases of varying physiological conditions, users may manually measure and calibrate the MA to control variations.
- However, manual calibration can be painful and time-consuming,
- Necessitating a device that performs fluid extraction or injection with minimal calibration and the disclosure aims to overcome any limitations mentioned above.
Technology
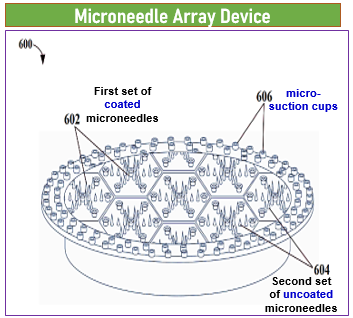
Method for manufacturing –Microneedle Array Device:
- Form first set of coated microneedles in first pattern
- Form second set of uncoated microneedles in second pattern, where second pattern is substantially different from first pattern
- Couple calibration module with second set of uncoated microneedles, where the calibration module includes optical sensor, calibration module configured to
- calibrate first signal received from first set of coated microneedles, based on second signal received from second set of uncoated microneedles
A number of the second set of micro-needles are 1/10th of a number of the first set of micro-needles
Key Features / Value Proposition
Increased lifetime:
- The device includes a set of coated microneedles that can be coated with pyrolytic carbon material to improve its lifetime and disposal.
Calibration-free:
- The device includes a second set of uncoated microneedles coupled with a calibration module, eliminating the need for manual calibration.
Reduced false-positive measurement:
- The device includes multiple microneedles disposed at different heights, ensuring a wider range of measurements from different user data points.
Painless adhesion and removal:
- The device includes micro-suction cups that form a negative pressure when contacted with user skin, facilitating painless adhesion and removal.
Questions about this Technology?
Contact For Licensing
sm-marketing@imail.iitm.ac.in
ipoffice2@iitm.ac.in
Research Lab
Prof. Parasuraman Swaminathan
Department of Metallurgical and Materials Engineering
Intellectual Property
IITM IDF Ref. 2597
Patent No: IN 540518
Technology Readiness Level
TRL- 3
Experimental proof of concept
