Industrial Consultancy & Sponsored Research (IC&SR) , IIT Madras
Technology Category/ Market
Technology:N-Methylpyrrolidinone hydroperoxide
Industry: Manufacturing/ Chemical
Applications: Chemical Synthesis.
Market: The global N-Methylpyrrolidinone market size is projected $1.34B by 2033 at a CAGR of 4.6% during 2023-2033.
Image Gallery
Problem Statement
- In the present era, there are many transition metal catalysts, known to activate hydroperoxides for oxy functionalization.
- Low stability, less commercial availability, explosive nature & difficult synthetic routes are some issues which limit the wide use of most of the peroxide-based epoxidizing reagents.
- Further, a few prior arts have discussed & found several issues including said issues.
- Hence, there is a need to mitigate above challenges.
- Present invention addresses above challenges in efficient manner.
Technology
- Present invention describes real synthetic method of N-Methyl-2- pyrrolidinone 5-hydroperoxide (NMPOOH) in organic chemistry.
- Present invention highlights the real synthetic use of NMPOOH in stereoselective epoxidation of electron deficient olefins.
- Importantly, the stereoselective epoxidation of electron deficient olefins reported here involve proposed mechanistic pathway in which the facial selectivity during epoxidation is brought about by secondary interactions involving the OH group in the substrate.
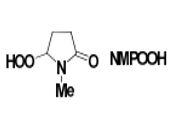
- The mechanism involves the hydroxy-directed syn epoxidation method by a base selected from K3PO4, K2CO3, Cs2CO3 or DBU.
Key Features/ Value Proposition
- Facilitates very good chemoselectivity in presence of other sensitive groups such as amine or sulfides.
- Practiced under benign experimental condition.
- Discriminates hetero-atoms like nitrogen & sulfur from oxidation.
- The NMPOOH generated in NMP provide the reagent as well as the reaction medium in the said epoxidation process.
- High diastereoselectivity is attained by secondary interactions involving OH present in the substrate.
- The NMPOOH-Cs2CO3 system is highly reactive & syn selective.
- Epoxidation by NMPOOH-Cs2CO3 reaction time ranges from 5-30 min with high yields in the range of 70-98%.
- The developed methodology has short reaction time, exhibits excellent diastereoselectivity in systems.
Questions about this Technology?
Contact for Licensing
sm-marketing@imail.iitm.ac.in
ipoffice2@iitm.ac.in
Research Lab
Prof. Muraleedharan K M
Department of Chemistry
Intellectual Property
IITM IDF Ref.:1149
Patent No. 308318
Technology Readiness Level
TRL – 3
Proof of Concept ready & validated
