Industrial Consultancy & Sponsored Research (IC&SR) , IIT Madras
A Process To Synthesize (ARYL)(HETEROARYL)Methanols
Categories for this Invention
Category: Chemistry & chemical Analysis, Drugs & Pharmaceutical Engineering
Industry: Catalysts and Chemical Manufacturing, Drugs & Pharmaceutical manufacturing
Applications: Drug Manufacturing
Market: The global methanol market is projected to grow from $28.74 B to $39.18 B at 4.5% CAGR during forecast period, 2021-2028.
Image Gallery
Problem Statement
- The (aryl)(heteroaryl)methanol derivatives are basic core in many drug molecules.
- Several efficient procedures were reported for the synthesis of aryl heteroaryl ketones by direct oxidation of C(sp3)–H bonds.
- Certain drawbacks are utilization of precious metal catalysts, pre-modified raw materials, sensitive chemical reagents.
- Moreover, no catalytic procedure is reported for the preparation of (aryl)(heteroaryl) methanols by a controlled oxidation of C(sp3)–H bonds of (aryl)(heteroaryl)methanes.
- So, it is of particular interest to develop an alternative and straight forward approach to synthesize (aryl)(heteroaryl)methanols.
Technology
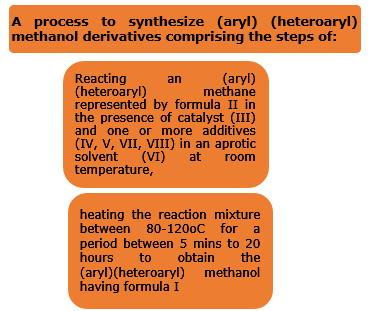
The present invention relates to a transition-metal-free process method to synthesize (aryl)(heteroaryl)methanol derivatives I from (aryl)(heteroaryl)methanes II through halogen bond-assisted C(sp3)-H bond activation strategy.
- Wherein Ar represents one of the groups independently selected from group comprising of:
- 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 3-methoxyphenyl, 4-ethylphenyl, 2-iodophenyl, phenyl, 4-methoxyphenyl, 4-(methylthio) phenyl, 3,5-dimethylphenyl, 4-tertbutylphenyl, 1-napthyl, 2-hydroxyphenyl, 4-chlorophenyl, 4-fluorophenyl, 2-thiophenyl, 1,1’-biphenyl, 4-bromophenyl, or 2-chlorophenyl
- catalyst is molecular iodine (III), One/more additives is selected from triphenylsilylchloride (IV), hydroiodic acid (V), trimethylsilylchloride (VII), triethylsilylchloride (VIII) and aprotic solvent is dimethylsulfoxide (VI).
- ‘N’ (nitrogen) embedded ring independently represents a heterocyclic ring selected from a group comprising of 2-pyridyl 4-pyridyl, and 2-benzo[d]thiazole, wherein X is methine group and Y is a heteroatom selected from ‘N’ or ‘S’ or ‘O’ and ‘n’ is an independent integer 0 or 1.
- The reaction stoichiometry the ratio between formula II, III, IV, V is 1:0.1:0.6: 0.2.
Key Features / Value Proposition
- This methodology particularly relates to an advantages preparation of wide range of medicinally important (aryl)(heteroaryl) methanol derivatives.
- The (aryl)(heteroaryl)methanol derivatives are basic core in many drug molecules like phenyl(pyridin-2-yl)methanol which is an anticonvulsant, (4- chlorophenyl) (pyridin-2-yl) methanol is an antihistamine intermediate of carbinoxamine & doxylamine & also serves as a key intermediate in chloropheniramine synthesis known for antirhinitis.
- It is derived by using polyaromatic group-substituted methane as a raw material, iodine as catalyst, silylchloride as additive and dimethyl sulfoxide as source of oxygen atom and a reaction medium in high yields & purity.
Questions about this Technology?
Contact For Licensing
sm-marketing@imail.iitm.ac.in
ipoffice2@iitm.ac.in
Research Lab
Prof. Govindasamy Sekar
Department of Chemistry
Intellectual Property
- IITM IDF Number: 2215
- IP Patent Number: 406714 (Granted)
Technology Readiness Level
TRL – 3
Proof of Concept
