Industrial Consultancy & Sponsored Research (IC&SR) , IIT Madras
Green Synthesis of Substituted 2-phenylimidazo-[1,2-a]pyridines
Technology Category/Market
Category – Chemicals, Pharmaceuticals Applications – Medicinal, material and natural product synthesis.
Industry – Pharmaceuticals, Drug Development, Medicinal Chemistry, Material Science, Green Chemistry and Sustainable Synthesis.
Market – The global specialty chemicals market size is estimated to reach USD 1151.32 billion by 2030 and expanding at a CAGR of 4.1% from 2022 to 2030.
Image
Problem Statement
- The structural reactivity of imidazo[1,2-a]pyridine core readily allows the introduction of new functional groups to modify into various pharmaceutically valuable compounds using transitionmetal-catalyzed reaction.
- The functionalization of imidazo[1,2-a]pyridine derivatives, currently relies heavily on transition metal-catalyzed reactions.
- These reactions often require expensive and toxic transition metal catalysts, such as gold, palladium, platinum, etc., and pre-functionalization of starting materials using aryl boronic acids, aryl halides, or aryl iodonium salts.
- Despite advances, there are very few reported methods for the direct C-H arylation of imidazo[1,2-a]pyridines, especially using aryl iodides as arylating agents, which could provide an efficient and direct route to target compounds.
- Therefore, there is a need to develop a reliable and scalable methodology that can achieve C(sp2)-H arylation of 2-phenylimidazo[1,2-a]pyridines in good yield under transition metal-free conditions using visible light exposure.
Technology
- The present invention relates to synthesis of medicinally valuable multi-substituted 2 phenylimidazo-[1,2-a]pyridines.
- The present invention provides a transition metal-free regioselective C-H arylation of 2 phenylimidazo-[1,2-a]pyridines using sustainable visible light as energy source through non-covalent interaction.
- A process for synthesis of 2,3-diphenylimidazo[1,2-a]pyridine derivatives (V) comprising the steps of:
- treating halobenzene of formula II in the presence of KOtBu in aprotic solvent at room temperature for 10-20 minutes.

- adding 2-phenylimidazo-[1,2-a]pyridines of formula I to obtain 2,3- diphenylimidazo[1,2-a]pyridine derivatives (V),
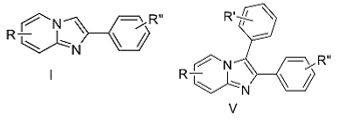
- wherein R represents an electron donating substituent,
- R’ represents electron-donating substituents and/or electron-withdrawing substituents,
- R’’ represents electron-donating substituents and/or electron-withdrawing substituents,
- X represents a halogen group selected from chloro, bromo, iodo, fluoro.
Key Features/Value Proposition
The present innovation elucidates the synthesis of 2,3-diphenylimidazo[1,2-a]pyridine (V) by treating commercially available starting material 2-phenylimidazo[1,2-a]pyridine
- with iodobenzene
- in the presence of KOtBu (4 equivalents)
- in degassed DMSO
- solvent under room temperature using visible light as sustainable energy source (Scheme 1).
- Transition Metal-Free: The current invention delineates toxic transition metal-free regioselective C-H arylation of 2-phenylimidazo-[1,2-a]pyridines using sustainable visible light as energy source through non-covalent interaction.
- Innovative Green Synthesis: Visible-light, sustainable energy, transition metal-free, halogen-bonding, imidazo-[1,2-a]pyridines, aza-heterocycles, , noncovalent interaction.
- One-Pot Synthesis: The innovative one-pot synthesis streamlines production, minimizing steps and resources required, exemplifying efficient and practical methodology.
- Energy Efficient: This is the first invention, which exposes the energy efficient and transition-metal free environmentally safer process for the arylation of 2-phenylimidazo-[1,2-a]pyridines derivatives.
- Regioselective Arylation: Achieves precise C-H arylation in a regioselective manner, enhancing product yield and reducing undesired byproducts.
- High Yield: Demonstrates 58-73% high product yield, ensuring efficient conversion of starting materials into desired products.
Questions about this Technology?
Contact for Licensing
Research Lab
Prof. Govindasamy Sekar
Department of Chemistry
Intellectual Property
- IITM IDF Ref. 2216
- IN 412957 – Patent Granted
Technology Readiness Level
TRL – 3
Proof of concept stage.
